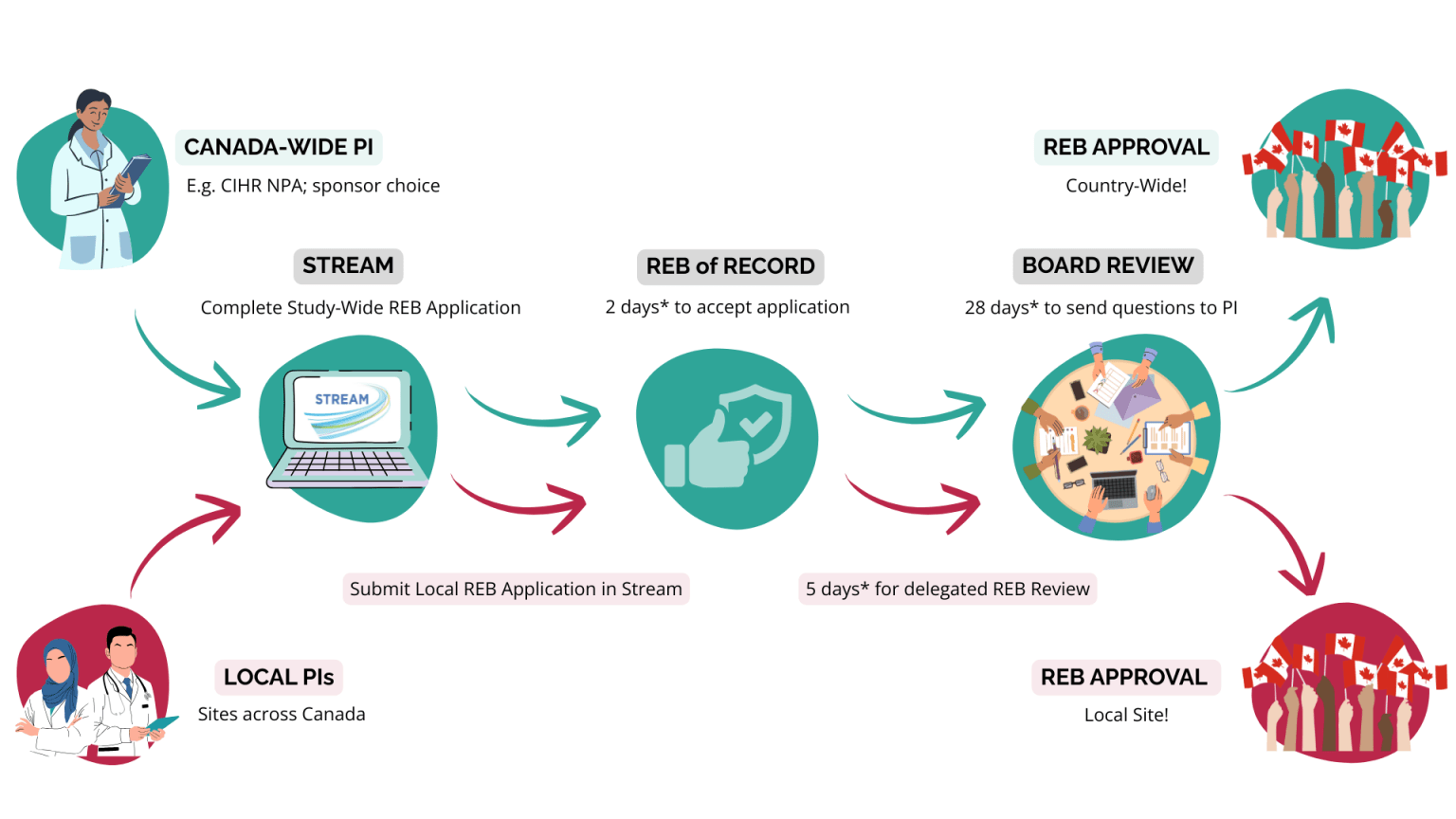
CanReview is Canada’s single research ethics review system. Purpose-built for pan-Canadian health research studies and clinical trials, it combines a distributed REB of Record model and Stream—an easy-to-use online platform—making it simple to submit or join multi-provincial studies, quickly add new sites to trials, and collaborate across Canada, all while maintaining the highest ethical standards.
With the CanReview system, there’s one research ethics submission, one review process, and one platform across Canada. A Principal Investigator (PI) submits a study-wide REB application through Stream, CanReview’s online platform. The assigned REB of Record accepts the application within 2 business days, and provides a decision with just 28 business days.
After receiving a study-wide approval, investigators across Canada can apply to join the study by submitting an application through Stream. The REB of Record assigned to that study will provide a decision within just 5 business days. Learn more about the application process here.
CanReview provides consistent, predictable research ethics review by ensuring timelines for both REBs and researchers that are strict yet fair.
For the initial country-wide review of the study protocol and study-wide materials:
If the REB determines that further clarification is needed, a 5-day cycle repeats: the PI has 5 business days to respond, and the REB has 5 business days to review. This cycle continues until the site receives approval.
For individual site approval:
If the REB determines that further clarification is needed, a 2-day cycle repeats: the PI has 2 business days respond, and the REB has 2 business days to review. This cycle continues until the site receives approval.
Quality Comes First
If an REB is unable to meet timelines (due to volume, meeting schedule, etc.), it will decline assignment so that the study can be reassigned. The REB will retain its place in the equitable rotation model.
‘Stop-the-Clock’ Measure
Sometimes applications are complex, and deeper considerations are needed. A ‘stop-the-clock’ measure will be available in cases where the review of a study or the responses by the investigators/research teams require additional time.
CanReview is for multi-site, pan-Canadian, health-based studies being conducted at any CanReview Participating Site.
Your study is right for CanReview if your institution is a Participating Site, and your study involves a minimum of 2 sites from 2 or more provinces or territories.
If your study will have participating sites in only one province and a single review system operates within that province, we recommend you use the provincial system for research ethics review. If you are looking to join a study, it must have received ethics approval in CanReview.
For industry-sponsored or supported trials, a service fee is charged for the provision of streamlined research ethics review. Canadian Biotech companies can capitalize on the value and efficiency of the CanReview system at a special discounted rate for multi-site, pan-Canadian clinical trials.
CanReview is committed to ensuring the highest ethical and quality standards. The following three programs meet and exceed all Canadian regulatory requirements for research ethics review and have been endorsed by CanReview’s cross-Canada REB Validation Tactical Team as pathways to become a participating REB in CanReview.
All studies in CanReview are assigned to a validated Research Ethics Board (REB) that has undertaken one of the above pathways. This REB of Record will review all submissions related to the study. REB assignment differs based on whether a study is industry-funded or investigator-initiated, and whether it would benefit from a specialized REB. In all cases, CanReview uses an equitable, ‘next-in-line’ model.
CanReview is committed to supporting jurisdictions where a local REB must engage in the research ethics review process (i.e. where required by provincial regulation or legislation). CanReview will support advocacy efforts to evolve legislation, and partner locally to co-develop proactive supports that enable engagement with CanReview. Those facilitated review mechanisms can include:
Review by selected CanReview Validated REB with invited Reviewers
A single record of a study within the system will be distributed to multiple REBs to undergo concurrent review or multiple provincial records of a study will be generated and distributed to matching provincial boards for review.
For studies approved through the system, REBs conducting a separate review can be provided access to the system to review applications, materials and comments made by the REB to facilitate their local review, both initially and throughout the study.
Data from CanReview can be exported to local systems (institutional or REB) to support local reviews and/or data collection through a variety of methods.
CanReview is a pan-Canadian collaboration supported by the ACT Consortium to enable a single research ethics review for multi-site clinical trials conducted across Canada, while ensuring the highest ethical standards. Collaborating with Research Ethics Boards, research teams, Indigenous community members, institutions and sponsors, patients and family partners and many others, we are co-developing a system to enhance efficiencies and increase Canada’s competitiveness, expand clinical trials to underserved, rural and remote locations, and promote equitable access to trial participation. Together, we are building a Canada-wide ethics review system that benefits all people in Canada.
CanReview leverages Stream, an adaptable, user-friendly web-based platform that facilitates a single research ethics review process for multi-site clinical trials. Leveraging this platform, CanReview will provide users with:
For studies that need review within province, CanReview can facilitate access to the online platform, enabling local REBs to review applications, materials, and comments from the REB of Record to streamline the process or employ other methods to respect local requirements. CanReview will continue to collaborate locally to co-develop proactive supports and advocate for legislative changes that support single ethics review for multi-site trials in Canada.
CanReview is building out committees and recruiting experienced and passionate people from across Canada’s clinical trials community for its key tactical teams – including people with lived experience. Together, these groups will collaborate to build a path forward, including working out the details for CanReview’s online platform.
If you’re interested in shaping Canada’s clinical trials ecosystem and supporting equitable access to timely clinical trials, get involved! Reach out to info@canreview.ca for more information.